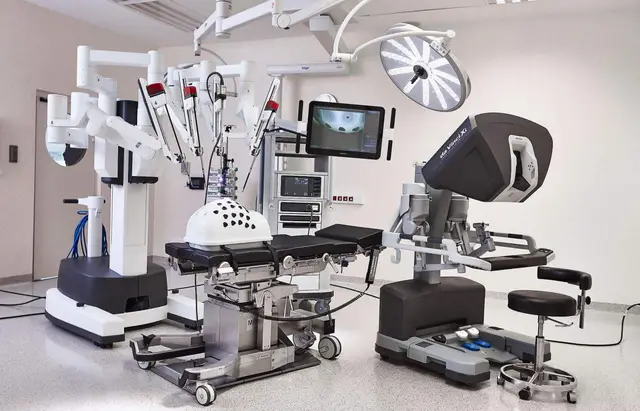
Technical Mechanism: From "Extending Hands" to "Precision Control"
The Da Vinci surgical system was first developed by the American company Intuitive Surgical, and its core structure includes a main console, robotic arm system, three-dimensional imaging system, and auxiliary energy platform. Doctors sit at the main console and control the robotic arms through a joystick for remote operation. Compared to traditional laparoscopic surgery, the advantages of the robotic system focus on three aspects:
Strong fine operation capability: The robotic arms can eliminate hand tremors, with a joint freedom of up to seven axes, capable of performing difficult suturing, dissection, and cutting;
Visual system is three-dimensional and clear: Provides 10x magnification and high-definition 3D views, facilitating the identification of vascular and nerve pathways and tissue interfaces;
More comfortable operating posture: Doctors do not need to stand for long periods, which can reduce fatigue and improve concentration.
Essentially, the Da Vinci surgical system is not an "automatic surgical robot"; it does not have autonomous operating capabilities but is a "doctor extension system" that emphasizes human-machine collaboration rather than replacement.
Taking transurethral prostatectomy as an example, traditional open surgery requires an incision of more than 15 centimeters, and laparoscopic surgical instruments are bulky, while robotic surgery can perform fine dissection and suturing in a very small space, reducing postoperative recovery time.
It is this concept of "precisely amplifying human capabilities" that makes surgical robots indispensable tools in certain specific fields.
The Real Boundaries of Indications and Promotion
Although robotic surgical systems have been registered and approved in multiple countries, their indications are still concentrated in limited subjects:
Urology: Radical prostatectomy, partial nephrectomy;
Gynecological tumors: Hysterectomy, extensive resection for cervical cancer;
Colorectal surgery: Low rectal cancer sphincter-preserving surgery;
Thoracic surgery: Mitral valve repair, lung segment resection;
Head and neck surgery: Thyroid and base of tongue surgery.
These fields share several common characteristics: complex anatomical structures, narrow operating spaces, and high functional preservation requirements. The robotic system can maximize its "minimally invasive precision" advantages in such procedures.
However, some hospitals, driven by "showing off skills" and economic incentives, have used robotic systems for routine minimally invasive surgeries such as cholecystectomy and appendectomy, leading to industry controversy. These surgeries are already very mature with laparoscopic techniques, and the robot has not brought significant advantages, but rather increased the burden on patients.
Data shows that in North America and Asia, the Da Vinci system is mainly used for cancer radical surgeries; however, in some regions, due to a lack of regulatory guidance, there are signs of "abuse" of robotic surgery. For example, in Taiwan, there have been complaints from the public about hospitals "over-recommending robotic surgery" without adequately explaining its costs and risks.
Therefore, although robotic surgery is a technological innovation, its promotion should still be limited to indications that "enhance value" to avoid resource waste and loss of trust in healthcare.
Dual Dimensions of Clinical Effectiveness and Data Analysis
Evaluating whether the robotic surgical system is "innovative" hinges on whether its clinical efficacy surpasses traditional methods.
From existing studies, robotic surgery has advantages in the following indicators:
Lower intraoperative blood loss: Due to precise operations, there is less vascular injury;
Shorter hospital stay: Higher degree of minimally invasive, faster recovery;
Better postoperative functional recovery: Such as a higher probability of preserving urinary and sexual function;
Lower conversion rate during surgery: That is, fewer cases of converting from minimally invasive to open surgery.
Taking prostate cancer as an example, multi-center studies in the United States have shown that patients undergoing robotic prostatectomy have better urinary control and sexual function preservation compared to open surgery; the tumor control rate is comparable one year post-surgery.
However, are these advantages enough to offset the high costs? There is still disagreement. A cost-effectiveness analysis from the UK's National Health Service (NHS) pointed out that the cost per robotic surgery is over £3,000 higher than traditional minimally invasive surgery, but there is no significant difference in long-term outcomes for non-high-risk cases.
Additionally, the learning curve for robots is relatively long, and new doctors do not have efficiency or accuracy advantages in their first 20 surgeries; instead, they may overlook intuitive judgment due to reliance on the system's magnified view, leading to cases of injury.
Therefore, the judgment that "robotics equals better" is not valid; its performance is highly dependent on the doctor's experience, equipment calibration, and the complexity of the cases.
Doctors' Experience and the Reality of the Learning Curve
At the doctor level, the introduction of robotic surgery has not only changed the way of operation but also reshaped professional skill training and thinking patterns.
First, doctors transition from "operators at the surgical table" to "dispatchers behind the console," needing to re-establish their perception of tissue tension, instrument feedback, and spatial construction.
Secondly, operating the Da Vinci system is not easy. Although the system has motion mapping logic, completing precise suturing, ligation, dissection, and other actions still requires repeated training. Statistics show that doctors who master complex robotic procedures typically need to complete 40 to 100 related surgeries to achieve a stable level.
This has also led to a "digital divide": younger doctors adapt quickly, while middle-aged and older doctors may experience resistance during the transition.
However, after the adaptation period, most doctors report that system operation is "easier" and "more controllable." Some gynecologists have stated that they used to need half a day of rest after five consecutive open surgeries, while the physical exertion after robotic surgery has significantly decreased.
In terms of career development, mastering robotic technology is gradually becoming a "promotion threshold," with large hospitals beginning to incorporate it into training assessments and promotion standards. This trend has also stimulated the rapid development of surgical robot training courses, simulation platforms, and digital teaching systems, promoting an overall transformation in surgical education.
Institutional Challenges and the Behind-the-Scenes Game of Regulatory Discrepancies
The popularization of robotic surgical systems involves multiple institutional issues such as equipment procurement, insurance payment, patient choice, and industry certification.
First, the pricing mechanism is unclear. Currently, most robotic surgeries in mainland China are not covered by medical insurance, with the cost of consumables for a single surgery ranging from 20,000 to 50,000 yuan, making it difficult for patients to judge whether the cost is "worth it." Some hospitals charge a robotic operation fee but do not clearly communicate the clinical benefit differences to patients, which can easily lead to disputes.
Second, the standards for doctor qualifications are inconsistent. Different hospitals have varying training and certification processes for robotic operators, with some doctors completing simulation training in a short period before performing actual surgeries, posing potential safety risks.
Third, the commercialization paths behind procurement. Some grassroots hospitals introduce robotic systems mainly for "brand effect" and "performance-driven" reasons rather than medical necessity, reflecting a distorted tension between medical resource allocation and performance systems.
The FDA in the United States and CE certification in the European Union implement strict approvals for robotic systems; China's National Medical Products Administration has also included surgical robots in the green channel for innovative device approvals. With the rise of domestic brands such as "TUMAI," "MicroPort," and "Tianzhihang," the Chinese surgical robot market is facing a new pattern of domestic substitution and price competition.
The core task of institutional construction is to clarify the clinical value boundaries of robotic surgery, establish transparent and fair application evaluation and payment mechanisms, and guide its technological development back to the "patient-centered" medical original intention.
Possible Paths to Reshape the Future of Surgery
Robotic surgery is not a "short-term trend"; it is redefining the essence of "surgery." In the future, surgical robots may deepen transformation in the following directions:
Enhanced "intraoperative perception" capabilities: Integrating pressure feedback, temperature sensing, and AI-assisted image recognition to make operations smarter;
Customization of surgical procedures and intelligent navigation: Combining preoperative CT, MRI, and other data for individualized preoperative planning to achieve "digital twin" simulated surgery;
Remote surgery: With the support of 5G and high-speed fiber networks, enabling experts to remotely control surgeries, enhancing regional medical equity;
Human-machine collaborative training platforms: Building virtual reality training systems to shorten doctors' learning cycles;
Miniaturization of surgical robots: Such as capsule robots and flexible robots entering narrow internal areas to expand operational boundaries.
The common goal of these development paths is to enable doctors to operate more precisely and easily while allowing patients to recover more safely and quickly.
Transitioning from "showing off skills" to "routine means," surgical robots are undergoing dual tests of technological rationality and social recognition. They are neither a myth nor a joke, but an extension of a medical tool. The key is not how dazzling the technology itself is, but whether it truly serves to enhance treatment outcomes and amplify doctors' capabilities.